
South Korea Consumer Chemical Products and Biocide Safety Management Law (K-BPR)
The Consumer Chemical Products and Biocide Safety Management Law (known as K-BPR) concerns the placing on the market and use of hazardous consumer chemical and biocidal products, which aims to protect public health and environment from these chemicals and products. This is achieved by assessment or notification of consumer chemical products’ risk, authorization of biocidal chemical substances and products and determination of biocidal chemical used products. K-BPR was taken into force on January 1, 2019, and its recent amendment was promulgated on March 24, and May 26, 2020. Its enforcement will start on January 1, 2021.
Main requirements of K-BPR
1. Consumer chemical product
Ministry of Environment (MoE) designated consumer chemical products that have a potential to expose hazardous chemicals to human and environments while its use at the home, office, and public facilities (“consumer chemical product subject to safety confirmation”). The consumer chemical products are categorized into two, of which requires either approval or notification.
1) Notification of consumer chemical products subject to safety confirmation
Any person who intends to manufacture or import a consumer chemical product that is subject to notification needs a product safety confirmation from concerned research institutes and notifies the confirmation results to Korea Environmental Industry & Technology Institute (KEITI) no later than 30 days since its confirmation.
2) Approval of consumer chemical products subject to safety confirmation
Any person who intends to manufacture or import a consumer chemical product that is subject to approval shall gain approval from MoE. It includes sterilizing materials such as anti-bacterial disinfectant for humidifiers, for preventing infectious diseases and other disinfection purposes, as well as insecticides, rodenticides, and hygiene repellents.
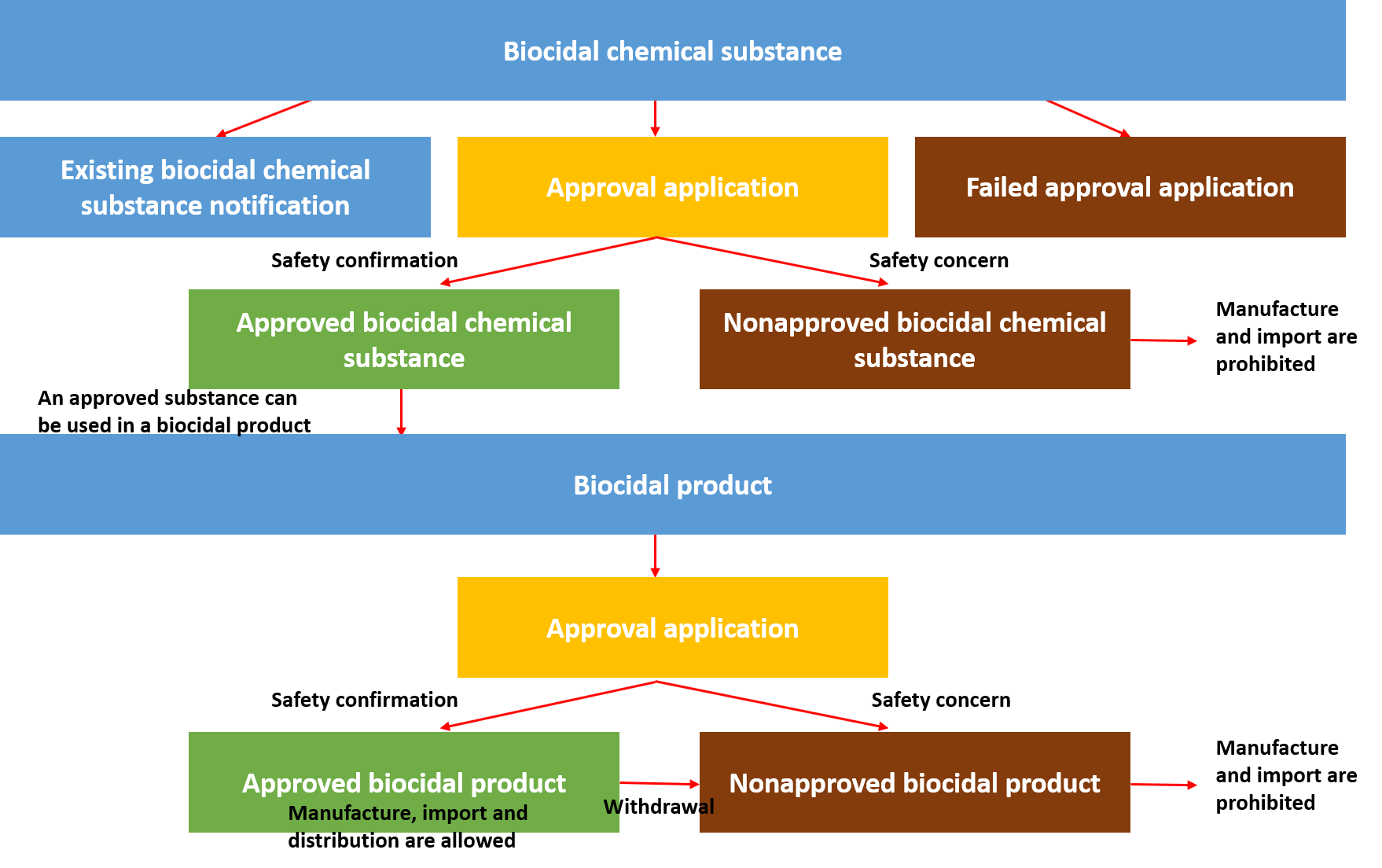
2. Biocides (Biocidal substance, biocidal product, biocidal treated product)
Any person who intends to manufacture or import biocides shall gain approval from MoE. Biocides under K-BPR refer to biocidal substances, biocidal products, biocidal treated products.
1) Biocidal substance
Biocidal substance refers to the chemical, natural material and microorganism to remove or control harmful organisms like pests and bacteria and make them harmless.
* Existing Biocidal Substance
Existing Biocidal Substance refers to a biocidal active substance included in biocidal products, which has placed on the market before December 31, 2018. By December 31, 2019, the list of 741 existing biocidal substances has been confirmed and varying substances have different grace periods.
* Biocidal substance other than the Existing Biocidal Substance
Any person who intends to manufacture and import biocidal substances requires approval from MoE. It applies to all biocidal substances except designated substances that have fewer risks, substances are used for research purposes and are subject to other laws.
2) Biocidal product
Biocidal product is defined as an article mainly used to remove harmful organisms, which is,
Any person who intends to manufacture and import biocidal products requires approval from MoE. Once a product is approved to be manufactured or imported, safety labelling for products concerned shall be stated.
Scope of K-BPR
| Within scope |
|
|---|---|
| Out of scope |
|
(1) ‘Consumer chemical product’ is defined as an article that has a potential of chemical exposure to human and environment while its use at the home, office, and public facilities etc.
(2) 'Biocidal substance’ refers to a chemical, natural material and microorganism to remove or control harmful organisms like pests and bacteria and make them harmless
(3) ‘Biocidal product’ is an article mainly used to remove harmful organisms, which is
(4) ‘Biocidal treated product’ refers to a product that uses biocidal products during the production process, but its main purpose is not to remove harmful organisms
Only representative (OR)
As per the recent amendment of K-BPR on March 24, 2020, the concept of Only Representative (OR) is included in the law, which will take into force on January 1, 2021. OR is an intermediary appointed by a foreign manufacturer based outside of South Korea to get an approval of consumer chemical products, biocidal substances, and biocidal products so that such chemicals and products can be imported in South Korea.
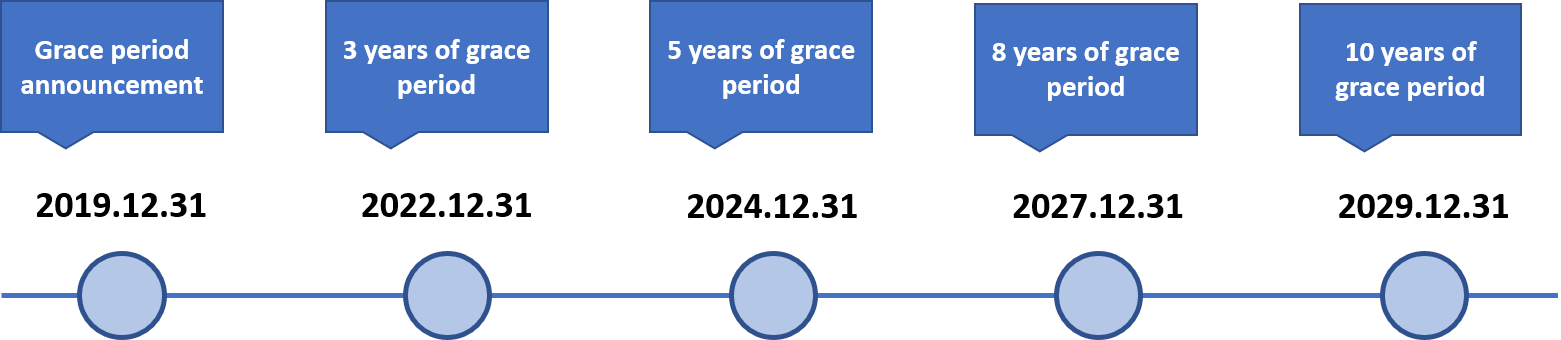
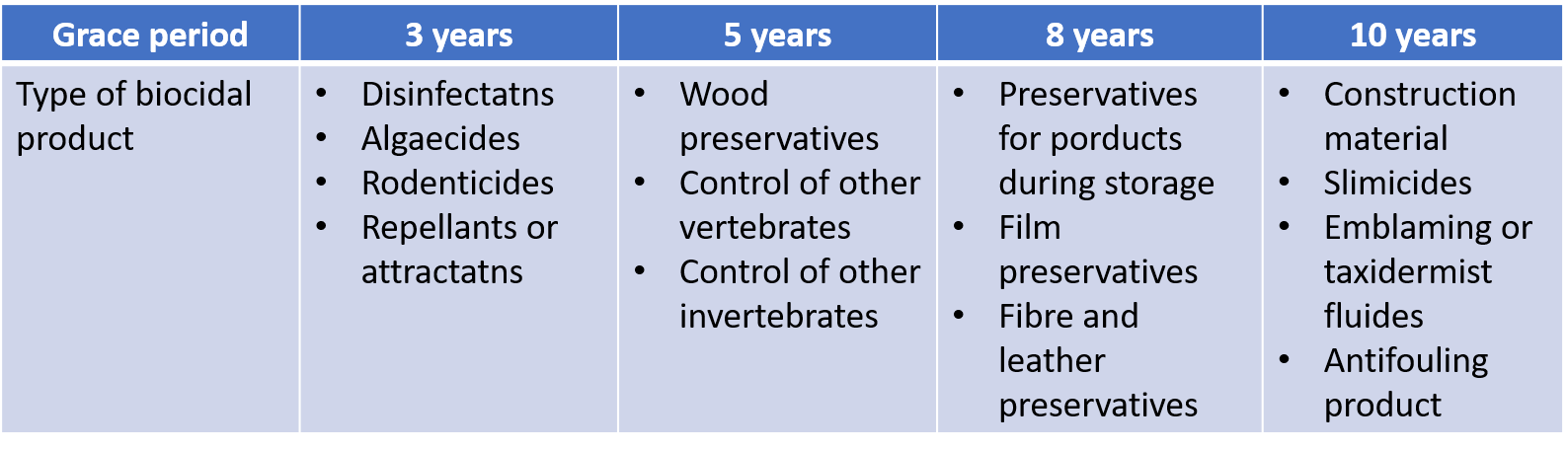
Grace period for the Existing Biocidal Substance
During the grace periods, any person who intends to manufacture or import biocides can continue to manufacture and import the biocides without approval from MoE once he or she submits an Existing Biocidal Substance notification to MoE. By December 31, 2020, for those companies who had reported their Existing Biocidal Substances, an approval plan (simplified version of approval application) shall be submitted in National Institute of Environmental Research (NIER).
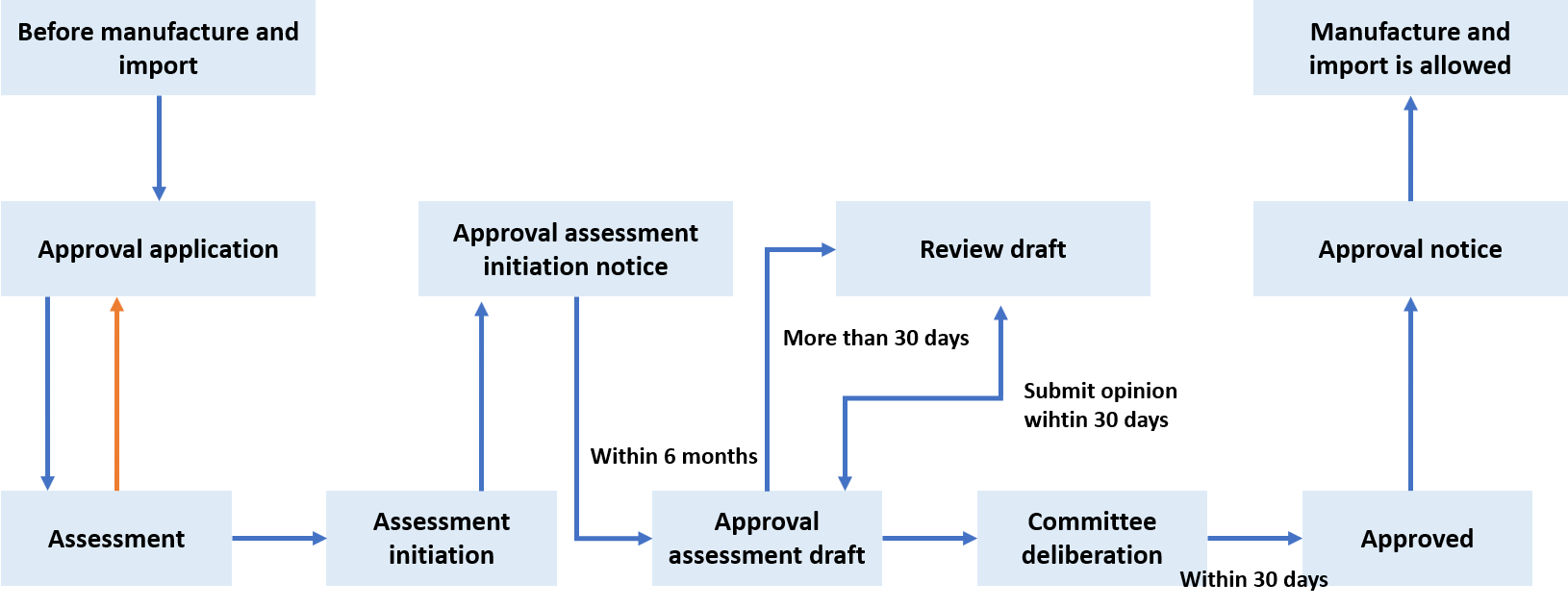
Biocides approval process
Biocides approval process begins with submitting approval application. Once the authority initiates the assessment, a notice informing approval assessment initiated will be sent to the applicant. In six months from the date of the notice, approval assessemnt result will come out and might require more information from the applicant. After going through committee deliberation, the approval application is finally approved and will be notified to the applicant. This process is described in the picture below.
For biocides use approval, then necessary documents are as follows.
1. Biocidal substance
An approval application form that includes applicant and biocides information (e.g. product name, material type etc.)
2. Biocidal product
An approval application form that includes applicant and biocides information (e.g. product name, material type etc.)
Expiration period of a biocides use approval
Each biocides use approval has expiration period.
1. Biocidal substance
| Expiration period | Criteria |
| 10 years |
|
| 7 years |
|
| 5 years |
|
(1) Approval criteria for a biocidal substance are eased when
2. Biocidal product
| Expiration periods | Criteria |
| 10 years |
|
| 5 years |
|
| 3 years |
|
(1) Approval criteria for a biocidal product are eased when
